Over the past decade, we have built an immunotherapy antibody (iTAb
TM) platform and obtained a series of global granted patents. The platform applies the mechanism of T-cell engager (TCE) to generate T cell activating bi- or tri-specific iTAb antibody (shown in Figure). One end of the iTAb molecule binds to tumor-associated antigen (TAA), or tumor-specific antigen (TSA) on a target tumor cell, while the other end of the molecule engages T cells. This leads to the activation of T cells and kills the target tumor cells, thus led to the clinical benefits to cancer patients.
Our bi-, tri-specific iTAb drug specifically binds to TAA or TSA on a tumor cell. When engaged with a T cell, it forms an immune synapsis between the tumor cell and the T cell. The T cell is then activated and releases granzymes and perforins leading to the tumor cell lysis. An activated T cell becomes a serial tumor cell killer and it can also proliferate to enhance the immune functions in patients and to sustain the long-term anti-tumor activities.
The bi-, tri-specific iTAb antibodies have unique properties. The potent T cell-engaging function results in super active targeting-kiling bioactivities. When targeting a TAA, iTAb molecules have 10
3 – 10
6-fold potency compared to the conventional antibodies. iTAb can kill a tumor cell expressing very low levels of TAA, such as tumor stem cells, and a tumor cell expressing a few TSA molecules per cell. The iTAb molecule without a Fc fragment avoids the potential off-target toxicities caused by Fc-associated binding, while maintaining the potent T cell engaging property. It also contains a structural stabilizing base designed with an excellent product stability profile and robust manufacturing process, omitting the scale-up requirement during commercial manufacturing.
Most cancer patients after immunotherapies still relapse. T cell exhaustion and antigen escape (or epitope loss) play critical roles for the treatment outcome for patients. We believe that iTAb products have the potential to overcome T cell exhaustion and antigen escape, thus leading to more durable responses and increased chance for patient cure.

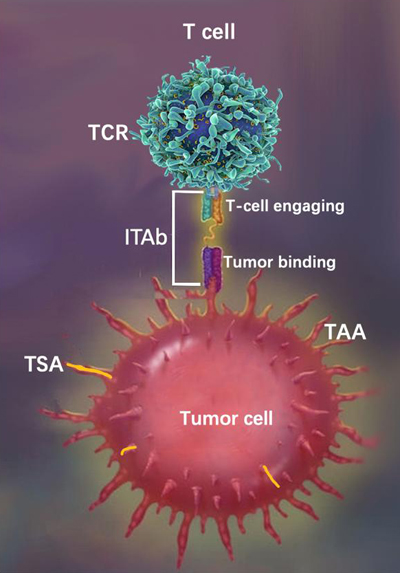
iTAb
TM: Immunotherapy antibody; TCR: T cell receptor; TAA: Tumor associated antigen; TSA: Tumor specific antigen.
The patent granted iTAb structure design is shown in the following figure:
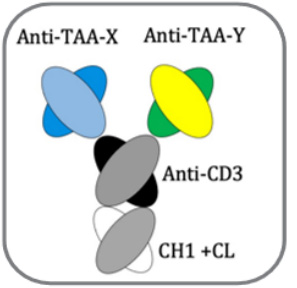
iTAb
TM : Immunotherapy antibody; TCR: T cell receptor; TAA: Tumor associated antigen; TSA: Tumor specific antigen.
The humanized CD3 antibody is cross-reactive to monkey with similar affinity.
The iTAb molecule can simultaneously bind two or more identical antigen epitopes or two or more different epitopes. It has super tumor killing bioactivity with EC50 at pM to nM. Due to its nanoantibody in size, an iTAb drug can efficiently enter a solid tumor, shown by the accumulation of activated infiltrating T cells within the tumor in animal models. iTAb is produced in CHO cells with robust manufacturing process, with an estimated commercial manufacturing scale at 200 L. iTAb in its liquid formulation shows an excellent stability, as it was within product Specifications after stored at 4oC for over 6 years.
T Cell Engager (TCE)
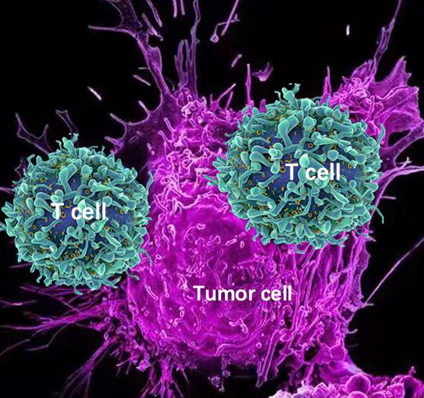
An illustration of activated T cells attacking a tumor cell. Once a T cell is engaged with a targeted tumor cell, the T cell is activated leading to the lysis of the tumor cell.
In the 1950s, Burnet and Thomas proposed that tumors express new antigenic arising from somatic mutation, and that these antigens are specifically targeted by the immune system. Over the past decades, this theory was gradually validated in cancer biology, animal models, and immunotherapy clinical trials. In recent years, immunotherapies have progressed rapidly and brought significant benefits to cancer patients, especially the immunotherapies based on T cell engager (TCE). The mechanism is that the T cell specifically engages the target tumor cell and becomes activated, leading to the target cell lysis. Immunotherapy was awarded as “scientific breakthrough” in journal of Science in 2013.
There are two main technologies for a T cell to engage a target tumor cell: First is Antibody, such as bi-, multi-specific T cell engaging antibodies; second is engineered T cells including CAR-T (chimeric antigen receptor T cell), or TCR-T (T cell receptor T cell) with an engineered T cell receptor. The common features of TCE are that they all take advantages of the potent tumor killing function of T cell in vivo. T cell-engaging antibodies and CAR-T bypass the restriction of MHC, which is a pathway required for a normal T cell activation. Once a T cell is specifically engaged with a target cell, an immune synapsis is formed between the two cells, leading to T cell activation and the release of perforins and granzymes, thus lysing the target cell.
T Cell Exhaustion
T cell exhaustion or dysfunction is very complicated and not well understood process. It is the main challenge facing cancer immunotherapy, especially for solid cancers. It seems that CD8 T cell dysfunction is the major contribution to I/O therapy relapse. The exhausted T cells seem to be derived from effector cells that retain the capacity to be long-lived. We have deployed two strategies to overcome T cell exhaustion.
CD8 TCE: We have developed CD8 T cell engagers which selectively bind and activate CD8 T cells.
“Hit-and-Run” dosing schedule: The iTAb drug shows a not very long half-life but with a desired duration of PD response in patients. The long-half-life, or continuously CD3 BsAb exposure can lead to T cell exhaustion with reduced clinical safety window.
Antigen Escape or Epitope Loss
Antigen escape is a common disease resistance mechanism to single-targeted therapies including CD19-CART, BCMA-CART, Blincyto, Rituximab etc. It was reported that down regulation/loss of CD19 antigen in 30–70% of patients who have recurrent disease after CD19 CART treatment.
iTAb products are able to target two or more antigens or epitopes simultaneously, thus reduce the probabilities of antigen escape.
 Over the past decade, we have built an immunotherapy antibody (iTAbTM) platform and obtained a series of global granted patents. The platform applies the mechanism of T-cell engager (TCE) to generate T cell activating bi- or tri-specific iTAb antibody (shown in Figure). One end of the iTAb molecule binds to tumor-associated antigen (TAA), or tumor-specific antigen (TSA) on a target tumor cell, while the other end of the molecule engages T cells. This leads to the activation of T cells and kills the target tumor cells, thus led to the clinical benefits to cancer patients.
Over the past decade, we have built an immunotherapy antibody (iTAbTM) platform and obtained a series of global granted patents. The platform applies the mechanism of T-cell engager (TCE) to generate T cell activating bi- or tri-specific iTAb antibody (shown in Figure). One end of the iTAb molecule binds to tumor-associated antigen (TAA), or tumor-specific antigen (TSA) on a target tumor cell, while the other end of the molecule engages T cells. This leads to the activation of T cells and kills the target tumor cells, thus led to the clinical benefits to cancer patients.





