A-337 is a EpCAM/CD3 bi-specific antibody with patent protection. It belongs to category 1 therapeutic biologicals according to NMPA guidelines and was generated by the iTAb
TM platform. The A-337 molecule has three binding domains; two domains bind to two EpCAM epitopes on a target epithelial cell, while the third domain binds to CD3 on a T cell, or a so-called T-cell engager. The T cell engagement leads to the formation of an immune synapsis between the T cell and target EpCAM-expressing tumor cell, resulting in the lysis of tumor cell. A-337 is produced in CHO cell in a serum-free medium and the manufacturing process is similar to that of conventional antibody manufacturing. A-337 drug product is a liquid formulation, which is stable over 42 months when stored at 4℃.
Epithelial cell adhesion molecule (EpCAM) is a transmembrane glycoprotein mediating Ca2+-independent cell-cell adhesion in epithelia. EpCAM is also involved in cell signaling, migration, proliferation, and differentiation. Additionally, EpCAM has oncogenic potential via its capacity to upregulate c-myc and cyclins A & E.EpCAM is over-expressed in most of epithelial tumors including colon cancer (97.7%), stomach cancer (90.7%), prostate cancer (87.2%), and lung cancer (63.7%).
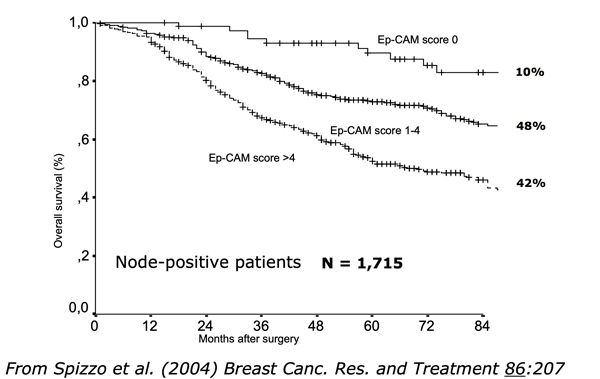
A-337 has the potential to treat EpCAM over-expressing various tumor types involving pancreas, lung, bile duct, colon, stomach, breast, bladders, and more.Approximately 80% epithelial tumors over-express EpCAM and the expression levels are inversely correlated to the poor patient survival and disease progression. For example, in breast cancer, patients with the higher level of EpCAM expression showed significant lower overall survival (shown in Figure).
The company received the IND approval from NMPA for A-337 in 2023. A Ph I clinical study is currently being initiated.



